
PASSAGEIROS PT htahelicopteros
HTA is a transparent and accountable process that can be used by decision makers and other stakeholders to support the decision-making process in health care at the policy level by providing evidence about given technologies It has been described as a bridge that connects the world of research to that of policy making. WHO Resolutions.

AS350B3PT htahelicopteros
A factsheet summarising the Health Technology Assessment Regulation, its governance and implementation is now available in 23 EU languages. 28 SEPTEMBER 2023.
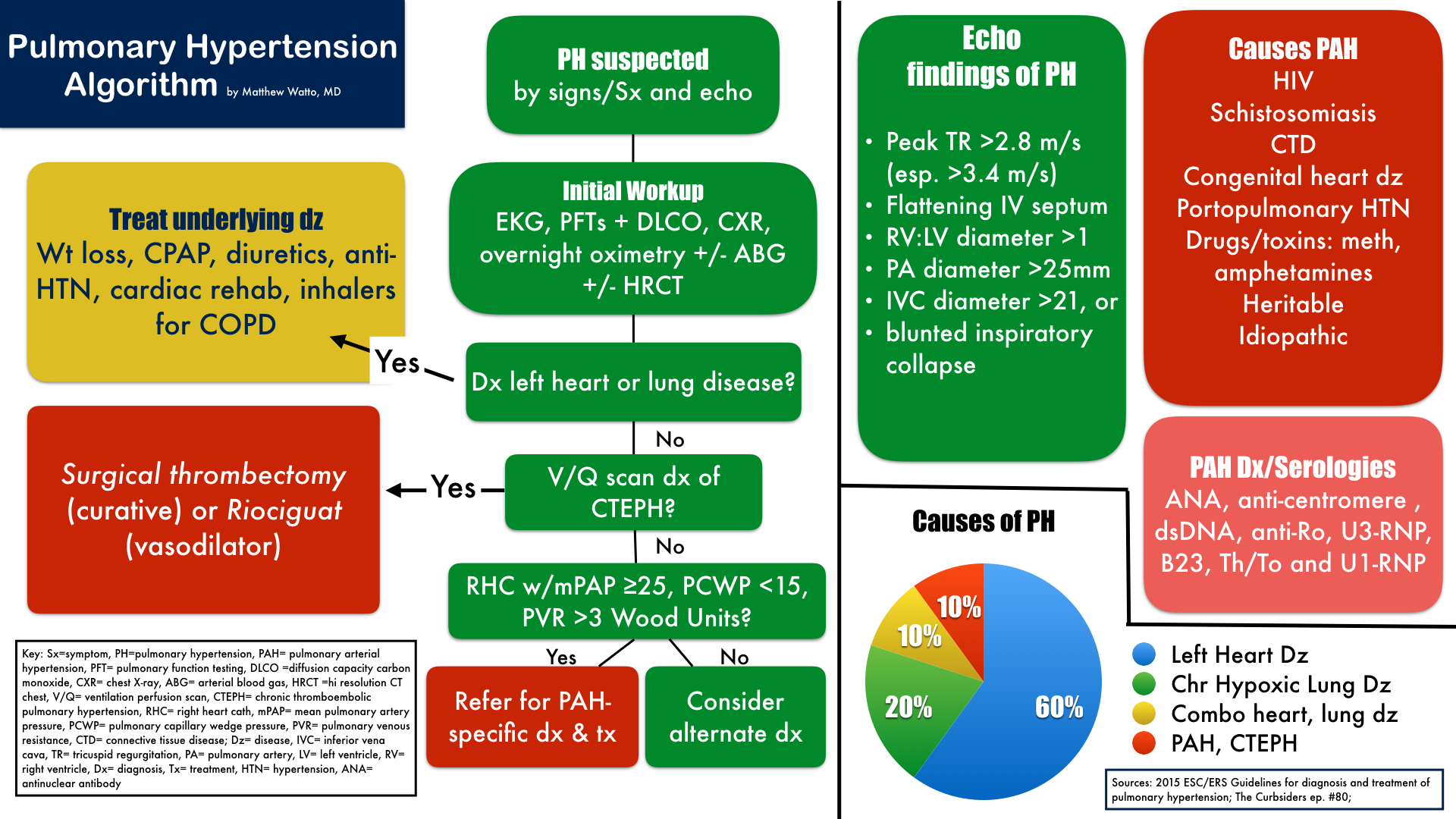
Pulmonary Hypertension Algorithm Matthew Watto, MD Diagnosis
Health Technology Assessment (HTA) is the systematic evaluation of properties, effects, and/or impacts of health care technology. It should include medical, social, ethical, and economic dimensions, and its main purpose is to inform decision-making in the health area. These assessments look at benefits and efficacy, clinical and technical.

PTHTA Vinicius Petris Flickr
High Tech Ancillaries Group is a group of companies that was established in 2018 and has quickly grown to become a leading manufacturer of rubber products, rubber materials, mold & dies, and a supplier of medical devices in the market. With five subsidiaries, including PT. High Tech Ancillaries Indonesia, PT. Arai Rubber Seal Indonesia, PT. Tri Tech Ancillaries Indonesia, PT. Dino Custom.

O que é a Hipertensão Arterial METIS
What is Health Technology Assessment? Health technology assessment (HTA) is a formal, systematic research process designed to synthesize and evaluate the existing evidence for a medical treatment or health delivery innovation (referred to as "technology" in subsequent sections of this document).

Curso de Atualização em HTA Update em Medicina
PT. HTA is committed to offering equal opportunities to all people without discrimination as to race, sex, nationality, ethnic or national origin, language, age, marital status, sexual orientation, religion or disability. PT. HTA does not tolerate harassment in the workplace in any form and remunerates fairly with respect to skill, performance.

Erasresidente Nueva guía de HTA
Asymmetrical dimethylarginine (ADMA) is an endogenous NOS inhibitor that is increased in those with HTN especially in patients with renal dysfunction. In the present review, the role of L-arginine, arginine transporters, and ADMA in the pathobiology of HTN and their potential clinical significance are discussed.

PASSAGEIROS PT htahelicopteros
PT. Sanggar Sarana Baja | perusahaan karoseri truk & alat berat Fabrikasi / Workshop: Millennium Industrial Estate, Jl. Millennium Raya blok F1 Tigaraksa, kab. Tangerang, Banten 15720 Telepon: (021) 5992150 PT. HTA Indonesia | produsen oil shield & komponen kendaraan dari bahan karet

Hawaii Technology Academy
With the PTT test, the reference range is between 25 and 33 seconds. As with the PT test, a higher PTT number means your blood is taking longer than usual to clot. A lower PTT number means that your blood is clotting faster than normal. There are several reasons why this might occur. Clotting Too Quickly.

PTHTA Vinicius Petris Flickr
HTA is proud to announce the release of 2800T, the first all-in-one autosampler in the market powered by AI capabilities. More Info. Product Selector. HTA S.R.L. VAT: IT02173320173. Headquarters. 77-79, via del Mella I-25131 Brescia (BS) | Italy. Phone +39 030 35 82 920. Email. [email protected].
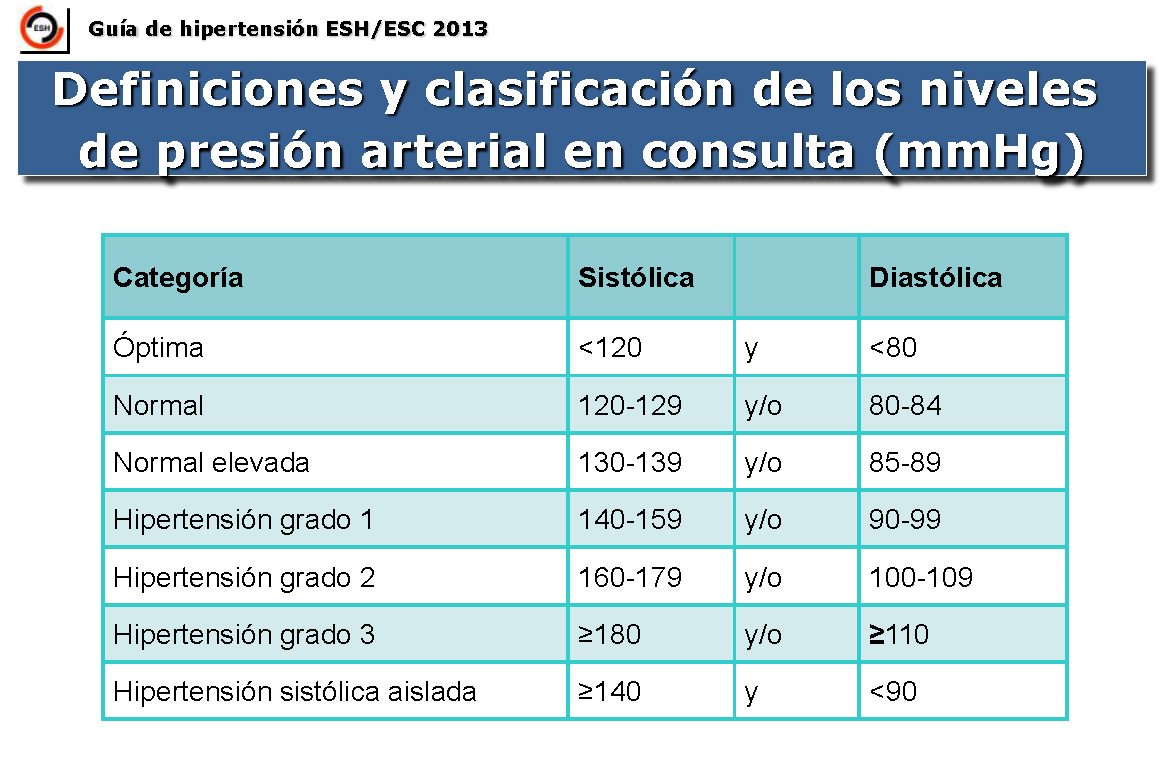
Erasresidente Nueva guía de HTA
The Regulation (EU) 2021/2282 on health technology assessment (HTAR) entered into force on 11 January 2022 and will apply from 12 January 2025.. In the preparatory phase for the implementation of the HTAR (January 2022 - January 2025), this webpage aims at informing national authorities, health technology developers and stakeholders about the development of implementing legislation in.

PTHTA Vinicius Petris Flickr
Aug 31, 2020. Blogs. Testing Times for Health Technology Assessment (HTA) With the ongoing coronavirus pandemic continuing to upend healthcare systems and economies around the world, health technology assessment (HTA) agencies have found themselves (unsurprisingly) in a difficult position. With healthcare priorities in many countries shifting.
PLAN HTA
A prothrombin time (PT) test uses blood samples to measure how quickly your blood forms a clot. If you're injured and bleeding, your body launches a step-by-step process that creates clots that stop the bleeding. That process involves proteins, called clotting factors or coagulation factors. Prothrombin is one of several clotting factors that.
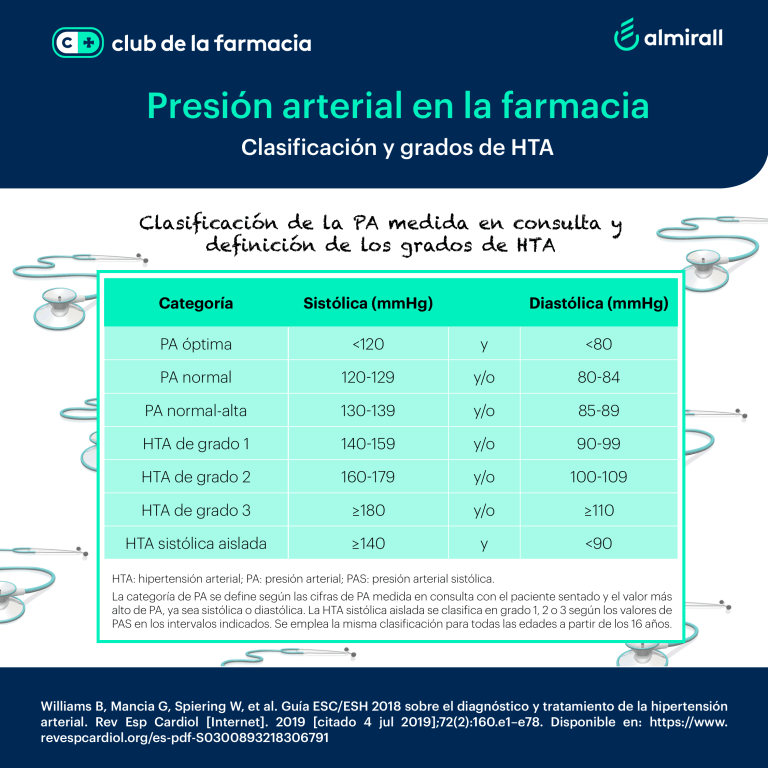
ClasificacionygradosdeHTA
Alprostadil for duct-dependent congenital heart disease. 2017. Clinical effectiveness and economic evaluation of cetuximab for metastatic colo-rectal cancer. Economic evaluation of bevacizumab for the additional treatment of metastatic colo-rectal cancer. Factors associated with the use of imatinib and nilotinib in patients with chronic.

PPT Factores de Riesgo C ardiovascular HTA PowerPoint Presentation
IPT Marks. Each of these three HTA products has an INAHTA Product Type (IPT) Mark, which is a small graphic, associated with it. It is recommended that this is placed on the front or inside page of the HTA so that anyone reviewing the document can immediately determine its product type. IPT Marks may be either unverified or verified.

PTHTA Vinicius Petris Flickr
High Tech Ancillaries Group is a group of companies that was established in 2018 and has quickly grown to become a leading manufacturer of rubber products, rubber materials, mold & dies, and a.